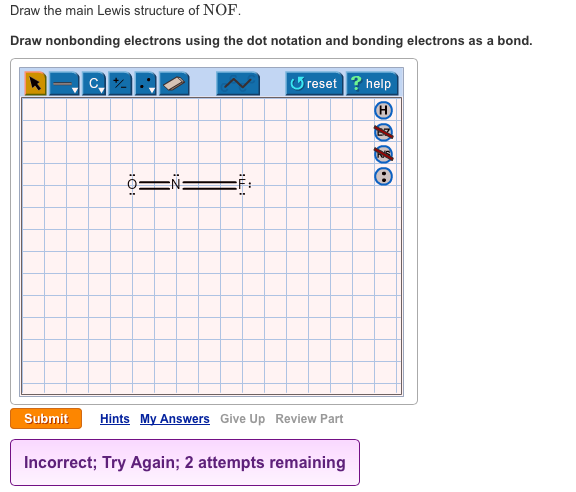
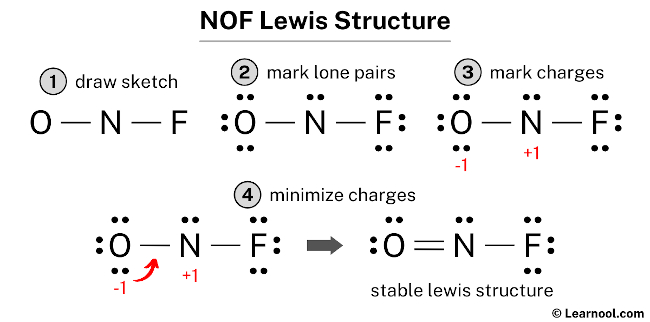
In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure. The lewis structure of NOF is Nitrogen is bonded to oxygen by double bond and fluorine by a single bond to complete the octet of elements.
Solved Draw The Main Lewis Structure Of Nof Draw Chegg Com
Nitrogen has five valence electrons.

. In the Lewis structure the central oxygen atom is bonded to two carbon atoms which have little or no electronegativity. Up to 256 cash back B Draw the main Lewis structure of NOF. To sketch the NaI Lewis structure by following these instructions.
As nitrogen is the least electronegative element amongst all the three atoms involved it is chosen as the central atom. A Lewis structure also helps to make a prediction about the geometry of a molecule. The six remaining hydrogen atoms in the molecule are evenly distributed among the carbon atoms.
The Lewis-dot structure are shown below. The outermost valence electrons of the NaF molecule must be understood while constructing the Lewis structure of the molecule. Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
90 THIS IS THE BEST ANSWER The most effective communication word rate is 120 words per minute. The first step is to sketch the Lewis structure of the NaF molecule to add valence electrons around the fluorine atom. Start filling in the gaps now.
What is the Lewis structure of Nitrosyl fluoride NOF. Lewis dot structures show all the valence electrons in an atom or molecule. O 6 - 3 ½4 1 The best Lewis structure is one that has the fewest formal charges the top structure.
The author doesnt offer facts statistics or specific examples but uses vague statements and sweeping generalizations. The NOF Lewis structure is very similar to NOCl and NOBr. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom.
Draw nonbonding electrons using the dot notation and bonding electrons as a bond. The formal charge on each atom is. In the NOF Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
Steps for Writing Lewis Structures. THIS USER ASKED The most effective communicators speak at a rate of _____ words per minute. XeO 2 F 2.
Draw the main lewis structure of nof. Lewis Structure of NaI for counting valence electrons around the terminal sodium atoms. N 5 4 - ½4 -1.
N 5 - 3 - ½4 0. Solution for Draw the main Lewis structure of NOF. It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
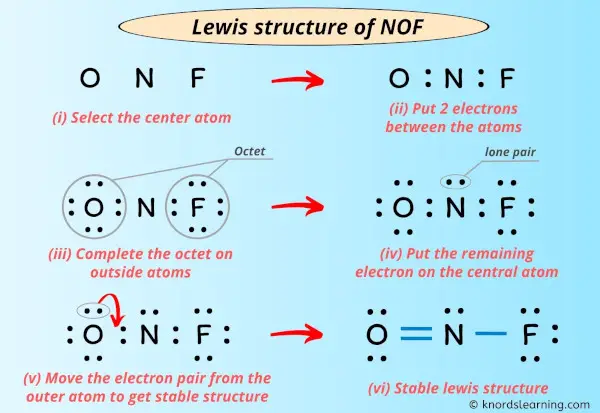
Draw the main Lewis structure of NOF. The Lewis structure of eqNOFeq nitrosyl fluoride is given below. The Oxygen and Fluorine atoms are placed on each side of the Nitrogen atom.
In the Lewis structure for NOF there are a total of 18 valence electrons. A Lewis structure is a graphic representation of the electron distribution around atoms. The given molecule is.
We can write two possible structures. Lewis Structure for NO 2-Nitrite ion Lewis structure of NO 2-ion is drawn in this tutorial. It determines the number of outermost valence electrons as well as the electrons engaged in the NaF ionic molecules bond formation.
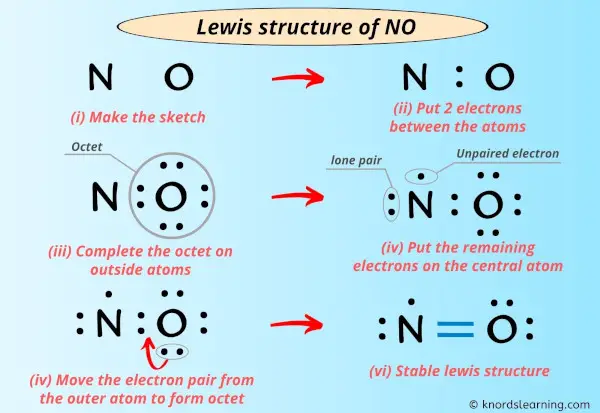
O 6 4 - ½4 0 Bottom structure. Experts are tested by Chegg as specialists in their subject area. Thus the Lewis structure of NO is.
NaI Lewis dot Structure by counting valence electrons on the iodine atom. Decide which is the central atom in the structure. The NOF Lewis structure is very similar to NOCl and NOBr.
Read More Which one of the following is an indicator that a source may not be accurate. Start your trial now. Lewis dot Structure for NaI generated from step-1 and step-2.
Drawing Lewis Structure of NOF Step 1. The writing is sub-par or contains errors. Draw the main lewis structure of nofnof.
First week only 499. To draw the Lewis structure of NOF we first need to choose a central atom. In the lewis structure for nof there are a total of 18 valence electrons.
Up to 256 cash back Draw the main Lewis structure of NOF. A Lewis structure is used to draw covalently bonded molecules as well as coordination compounds. Draw the nonbonding electron using the dot notation and bonding electrons as a bond.
The valence electrons are shown by dot. Check the formal charges to be sure that each atom has a formal charge of zero. Draw nonbonding electrons using the dot notation and bonding electrons as a bond.
Determine the number of bonding electrons and the number of nonbonding electrons in the structure of BeF2. Weve got the study and writing resources you need for your assignments. The electron dot structure of the NaF molecule is also known as the NaF Lewis structure.

Nof Lewis Structure Geometry Hybridization And Polarity Techiescientist

Draw The Lewis Structure Of Nof Nitrosyl Fluoride Youtube
Lewis Structure Of Nof With 6 Simple Steps To Draw

Nof Lewis Structure How To Draw The Dot Structure For Nof Homeworkavid
Lewis Structure Of No With 5 Simple Steps To Draw
Lewis Structure Of Nof With 6 Simple Steps To Draw

Nof Lewis Structure How To Draw The Lewis Structure For Nof Youtube
0 comments
Post a Comment